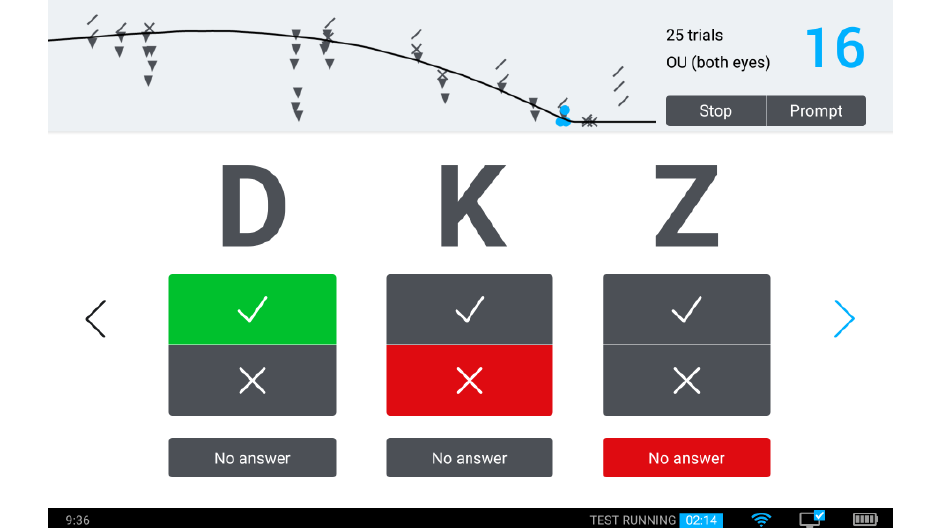
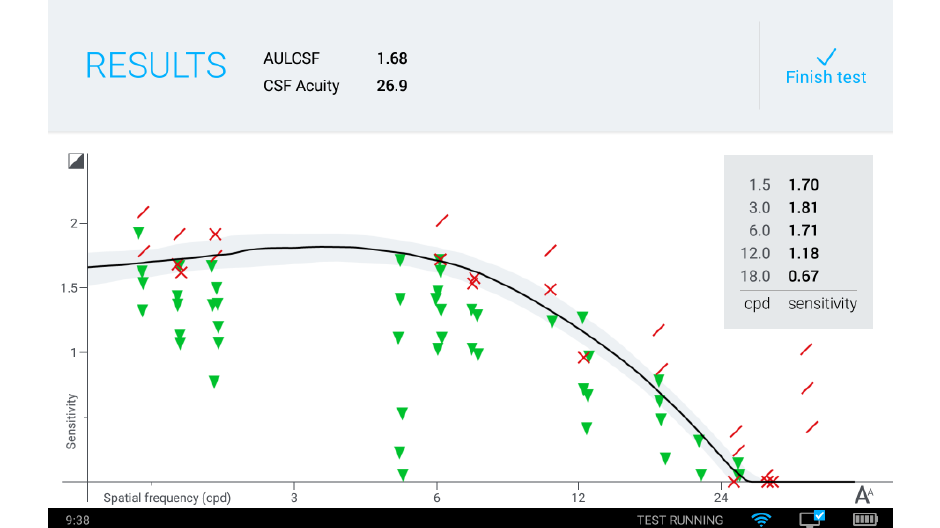
We’re offering a system for clinical testing that takes advantage of the qCSF algorithm and delivers it to the marketplace: the Manifold®. The Manifold® is a distance vision clinical testing system that includes several parts: a display, cart, and handheld remote control, with software components that include the qCSF algorithm.
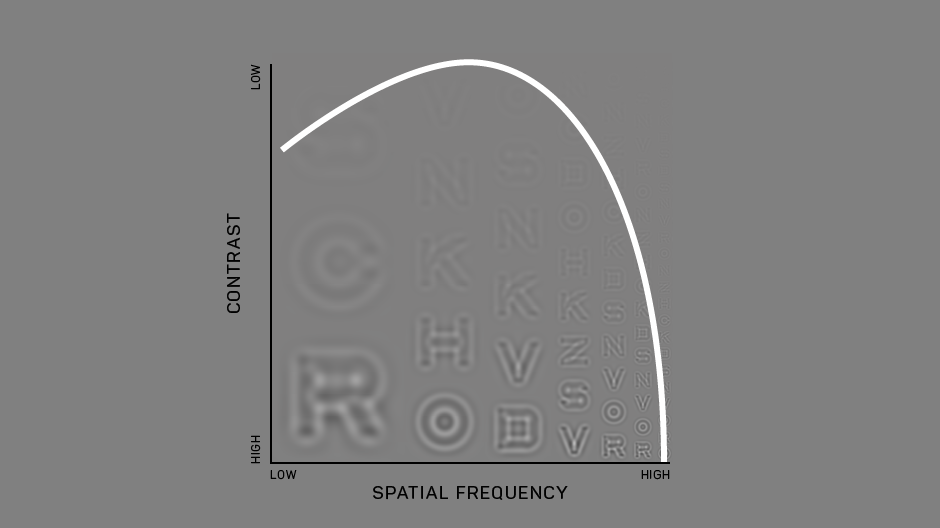
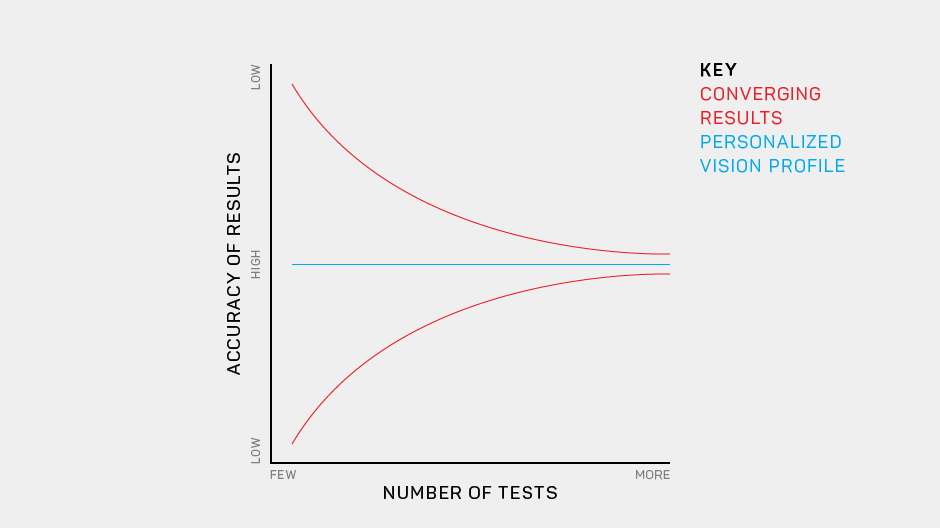
Our development of the Manifold® has refined the test to improve usability and comfort, while maintaining laboratory-grade precision. Our first research version used sine-wave gratings similar to other assessments of the contrast sensitivity function, with a high guessing rate (50%) and lower statistical efficiency. To translate this work to a clinical tool we have tapped previous work on spatial vision. The Manifold® uses the set of Sloan optotypes, which are then bandpass-filtered with a raised cosine window, with peak frequency 4 cycles per letter. The hybrid letter-grating stimulus that results provides the best of both worlds: the pattern is easily identifiable as a letter, but also exhibits the narrow-band frequency information of a grating. Another advantage of the Sloan set is the reduction of the guessing rate (1 of 10 = 10%), which improves test efficiency.
We seeded prototypes of the Manifold® to research and clinical labs in the United States and Germany. Our research partners—prominent clinicians from Harvard Medical School, Johns Hopkins, Hamburg-Eppendorf, Wright-Patterson Air Force Base, Navy Surgical Medical Center, Kellogg Eye Center, Retina Foundation of the Southwest, and Nova Southeastern College of Optometry—helped us gather real-world data to validate the device and method.
The commercially available first-generation Manifold Contrast Vision Meter was successfully used in more than 20 completed and ongoing clinical trials. Its successor, the Manifold Vision Meter, additionally offers quantitative Visual Acuity assessments, a wider spatial frequency range for testing from low-vision IRD patients to healthy controls, and enhanced usability with the unique protocol management feature. By now, the Manifold® has been deployed in international Phase II and Phase III and provided secondary and primary endpoints to support drug development across a number of indications including diabetic retinopathy, intermediate AMD, and geographic atrophy.
We believe AST products will become a new standard: a rapid and precise way to assess and monitor visual health over time.